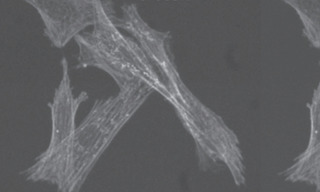
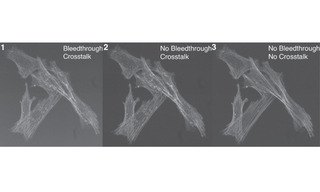
The origins of bleedthrough and crosstalk, two common confounding problems in fluorescence microscopy, are quite often confused. The following three 40X images of FITC-labeled actin and Cy3-labeled mitochondria serve to demonstrate the different manifestations of, and remedies for, bleedthrough and crosstalk.
Image 1. SPECTRA X Light Engine
Cyan channel excitation, 485/25 filter, Semrock LED-DA/FI/ TR/Cy5-4X quad polychroic and emitter
Both bleedthrough and crosstalk are present in this image. Bleedthrough is manifested by the relatively high extracellular gray level in the image (compare with Image 2 where bleedthrough has been eliminated).
Image 2. SPECTRA X Light Engine
Cyan channel excitation, 475/28 filter, Semrock LED-DA/FI/ TR/Cy5-4X quad polychroic and emitter
Bleedthrough has been eliminated by changing the excitation filter to one with a transmission band that does not intersect with those of the quad emitter. The cause of bleedthrough is transmission of excitation light through the emitter to the camera. Crosstalk, manifested by detection of perinuclear mitochondrial fluorescence, is still present in this image (compare with Image 3 where crosstalk has been eliminated to produce an actin-specific image).
Image 3. SPECTRA X Light Engine
Cyan channel excitation, 475/28 filter, Semrock single band FITC dichroic and emitter
Fundamentally, crosstalk is due to excitation and emission spectra of fluorophores such as FITC and Cy3 not being confined to discrete wavelength ranges. Even though Cy3 fluorescence is optimally excited by green (~550 nm) light, it is also excited by cyan (475 nm) light to a sufficient extent to be readily detectable in Image 2. The crosstalk signal is eliminated by changing the quad band polychroic and emitter for a single band dichroic and emitter to achieve increased blocking on the emission side of the detection system. This solution is a compromise, as it produces an increase in resolution at the expense of speed.
- Feb 13, 2018