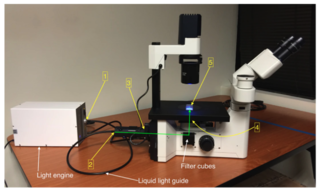
Figure 1: Coupling of a solid-state Light Engine to an inverted fluorescence microscope. Locations 1–5 marked in yellow correspond to the points in the light path used for throughput measurements reported in the table. The green line shows the direction of the light path from the collimating adapter input to the sample plane.
Increasingly, sophisticated Light Engines with an array of solid-state technologies are being used in fluorescence microscopy. The design of these microscope light sources overcomes significant limitations of mercury and metal halide arc lamps, enabling impressive performance far surpassing that of those earlier illumination options. Solid-state Light Engines provide high signal-to-noise ratios (SNRs) and resolution, low detection limits, and enhanced sensitivity and stability for fluorescence microscopy applications—as well as high-quality data, longevity, and cost savings.
However, for the value of that performance to be realized, the output of the illuminator must be propagated to the sample plane of the microscope.1,2 An analysis of the light path connecting the Light Engine to the microscope sample plane provides a framework not only for optimizing instrument performance, but also for troubleshooting illumination problems.
The path to truth
When deficiency in fluorescence excitation at the sample plane is discovered, it seems appropriate (and it is common) to attribute the problem to the light source. But such attribution is commonly inappropriate because, in fact, all of the intervening components along the path are potentially implicated. Actually solving the issue requires a thoughtful review of contributions made by all the optical components that transfer illuminator output to the microscope (see figure), and thus affect overall system throughput.
The table presents a stepwise analysis of light throughput from a solid-state Light Engine to the sample plane of a microscope. The measured fluxes at the sample plane using the 10X objective (Location 5A) correspond to irradiance levels of 15–50 mW/mm2, well within the required range for widefield fluorescence microscopy (1–100 mW/mm2).
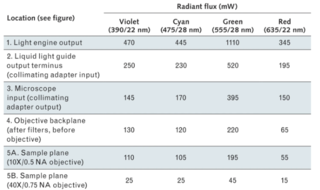
Figure 2: A Coherent FieldMax II-TO power meter, connected to a PM3 thermopile detector, produced the power measurements shown above (precision ±5 mW). The light source was a Lumencor SPECTRA X Light Engine with internal 391/22 nm, 475/28 nm, 555/28 nm, and 635/22nm (Center wavelength/full-width half-maximum bandwidth) excitation filters. Output from the Light Engine was delivered to the microscope (Nikon Ti) via a 3-mm-diameter liquid light quite and a Lumencor collimating adapter, and was then directed to the sample plane by a Semrock FF409/493/573/652-Di02 quad dichroic and through 10X/0.5 NA or 40X/0.75 NA objectives.
For fluorescence microscopy applications, the irradiance required to generate a certain amount of fluorescence emission is not the same at all wavelengths. This is because fluorescence emission intensity depends on the number of photons available for absorption by the fluorophore, which increases with wavelength for a constant level of irradiance. The irradiance required to generate a certain fluorescence intensity level with 375 nm excitation light is therefore double that required at 750 nm (2 × 375 nm), to the extent that other factors remain constant. Consequently, the lower radiant flux levels in the red spectral region (635/22 nm; see far right column in the table) are less detrimental than they might seem in the context of fluorescence microscopy.
Although the radiant flux levels shown in the table are more than sufficient for conventional widefield fluorescence microscopy, opportunities for improvement do exist. Such improvements are directed towards two main application requirements. First, to acquire more images in a shorter space of time without compromising SNR characteristics requires higher radiant flux from the light source coupled to shorter camera exposure times. Second, many techniques for increasing the spatial resolution of widefield fluorescence microscopy involve spatially filtering the illumination. The loss incurred by spatial filtering must be compensated by increasing the output of the light source or improving its coupling to the microscope, or both.
Getting to better
As an illustrative exercise, let’s briefly examine some ways in which these improvements can be achieved, referring to the light path stages identified in the table.
Light engine. Improvements in output power (particularly in terms of irradiance) can be achieved by using solid-state diode laser light sources, as opposed to the non-coherent sources incorporated in the SPECTRA X Light Engine used in this example.
Liquid light guide. A liquid light guide provides benefits in terms of vibrational isolation of the Light Engine from the microscope. It can also allow the light source to be situated remotely, away from the often-congested space adjacent to the microscope. However, these benefits can come at the cost of 35–50% of the Light Engine output—though with a sufficiently powerful Light Engine, the significance of these losses may be ameliorated. And although a liquid light guide with a larger diameter (e.g., 5 mm instead of 3 mm) delivers a substantial increase in radiant flux, this generally requires re-engineering of the microscope light path to propagate the increase to the sample plane.
Collimating adapter. Collimators incorporating fly-eye lenses provide superior light throughput and spatial uniformity compared to conventional designs based on an achromatic lens.
Objective. In epifluorescence microscopy, the detected fluorescence intensity scales with the fourth power of the numerical aperture (NA) of the objective lens. Therefore, the preferred objective is one with the highest numerical aperture that provides adequate working distance.
Illumination for fluorescence microscopy has undergone a paradigm shift. Traditional arc lamps have many limitations, including the fact that they contain toxic mercury vapor. Traditional LEDs, on the other hand, underserve optical power requirements. Solid-state Light Engines solve for these problems and and a viable alternative when insufficient light is discovered as an additional problem. But following the steps of the analysis presented above will help microscopists understand the details of illuminator-to-microscope coupling, and gain confidence in finding the true source of such issues. The hope is that it will also assist researchers in taking maximum advantage of the performance offered by modern solid-state Light Engines.
- Apr 28, 2021